Breast Exam by IDUS™
IDUS™ has significant potential in different areas of medicine. One disruptive and robust application in breast care has the largest growing markets in the Asia Pacific, followed by the US, Europe and the Middle-East. The proprietary and groundbreaking technology is hybrid and fundamentally transforms the universe of breast care by earlier, better, easier, cheaper and radiation-free detection and location of microcalcification, a clinically-proven marker directly associated with breast cancer. The marker’s early detection and location allow for effective follow-up monitoring and preemptive excision of the premalignant lesions before the growth of a cancerous tumor in the breast.
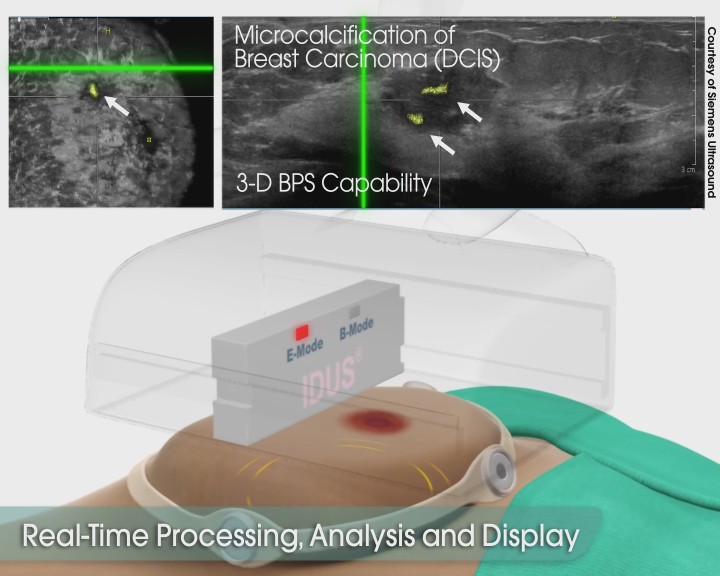
IDUS™ is particularly suited for the next generation of 3D whole breast ultrasound scanning systems. With the patient in a supine position, a hands-free automated ultrasound scanner is placed above the breast, gently pressed downward. A sequence of B-mode and IDUS™ images is then acquired, processed and displayed. The result is a complete 3D volumetric image of the whole breast supplemented by color coded IDUS™ 3D indicators for grouped or clustered microcalcifications based on their unique response signature(s). Note, microcalcifications are invisible on conventional ultrasound or MRI. IDUS™ data are integrated in real-time and interleaved with the traditional B-mode images providing complete 2D/3D scans essential to clinicians for better screening, follow-up monitoring and enhanced diagnosis in a manner which is safe, comfortable, radiation-free and cost-effective. A screening procedure using IDUS™ can be completed in less than 10 minutes.
IDUS™ - Supine Exam with Hands-free Automated 3D Scanner
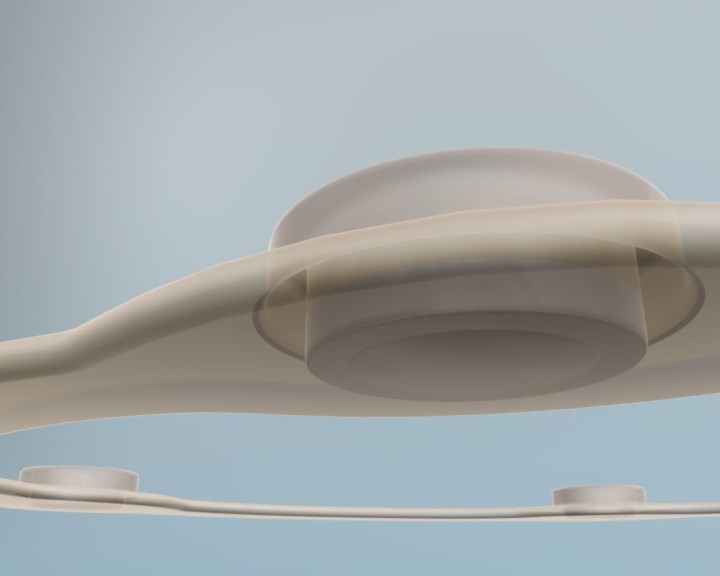
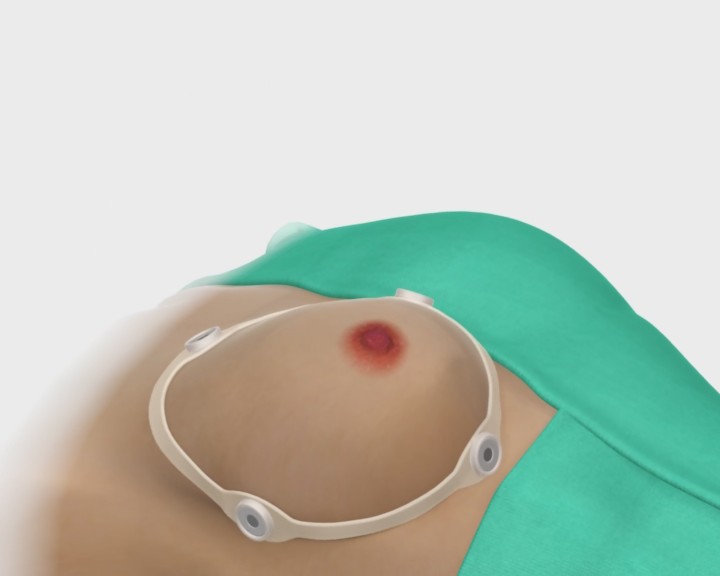
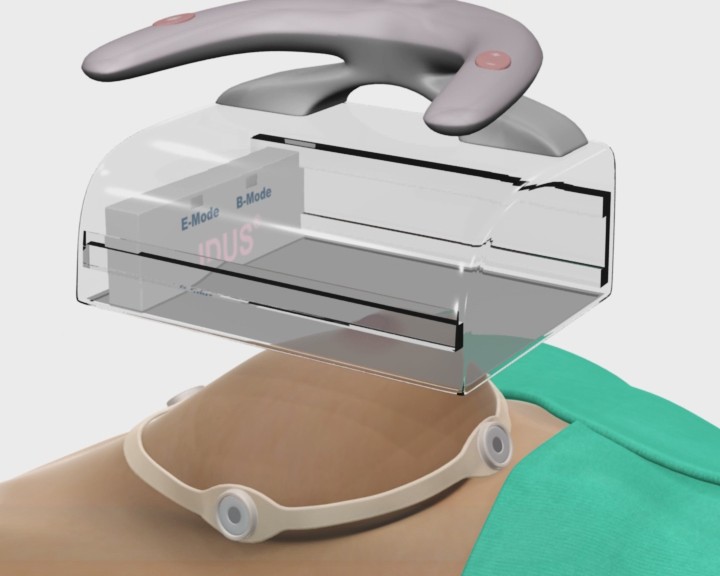
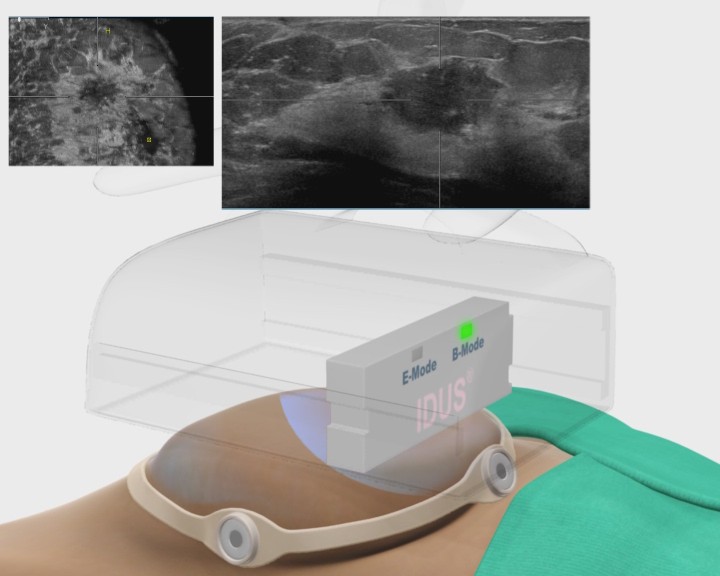
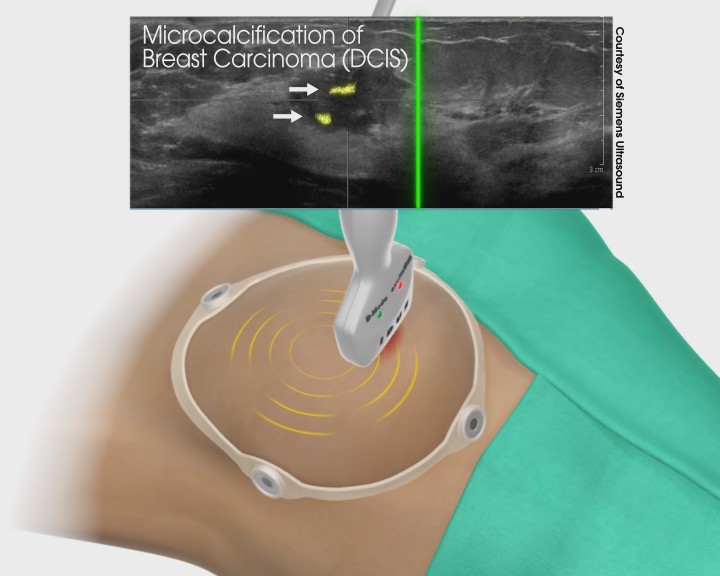
IDUS™ is also compatible with ultrasound systems using hand-held probes, providing superior detection sensitivity and precision guided biopsy.
IDUS™ - Supine Exam with Handheld Transducer
The competitive advantage of IDUS™ is in its use of widespread, ionizing-radiation-free, real-time and cost-effective ultrasound, which is patient friendly, user-intuitive, time- effective, cost-effective and reimbursable. Its economic advantage benefits the patients, the providers and the payers. Its social advantage benefits the society in general and women of all age, race, ethnicity and origin, in particular.
IDUS™ is ultrasound-based and unlike any other modality used in breast care:
- It has two modes of operation for screening & diagnosis:
- Passive imaging; and
- Active stimulation for real-time target acquisition and 3D positioning.
- It delivers superior detection sensitivity for enhanced screening and diagnostic confidence.
- It is designed for early and cost-effective screening for prevention, instead of late diagnosis and costly treatment.
- It has no ionizing radiation, harmful side-effects or discomfort.
- It has no need for contrast agents.
- It is real-time with exams lasting in less than 10 minutes.
- It is the only breast screening method for women under 40-45 years of age.
- It is ideally suited for women of all ages with dense or fatty breasts.
- It is practically suited for pregnant, lactating women or those with radiation therapy.
- It has very small footprint and is logistically suited for hospitals, imaging centers, woman’s health units and private doctor offices.
- It is expected for authorization by the FDA as a designated Class II device (510(k)) based on an existing predicate device.
- It has no burden on the clinical workflow and is projected to use two existing reimbursement (CPT) codes.
IDUS™ is aimed at realizing the true potential of ultrasound. It has significant potential with substantially growing, scalable, expanding and unmet markets. In more than 16,000 hours of research and development, IDUS™ has already proven pre-clinical validity. Prior to its use in breast care, IDUS™ was successfully implemented in precision and preemptive detection of structural flaws in implanted mechanical heart valves (see below), thus eliminating fatal consequences.
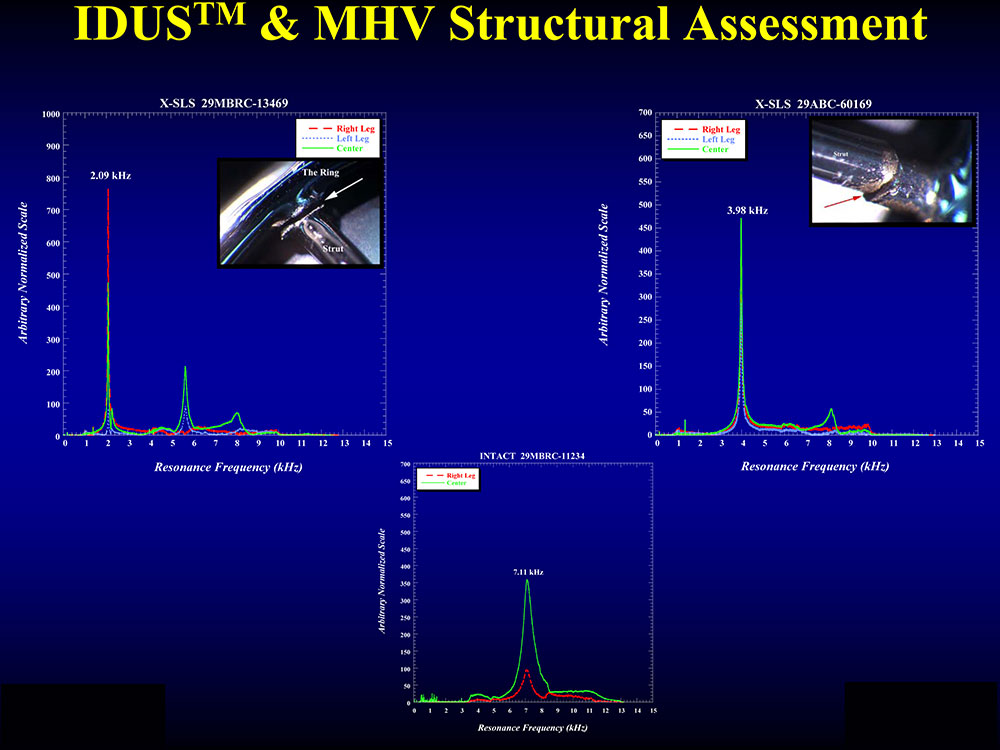
IDUS™ is protected by 14 issued and pending patents.
IDUS™ has additional applications, including:
- A. Precision biopsy guidance
- B. Site-specific drug delivery
- C. Cardiac navigation
- D. Neuro-stimulation
- E. Orthopedic


